
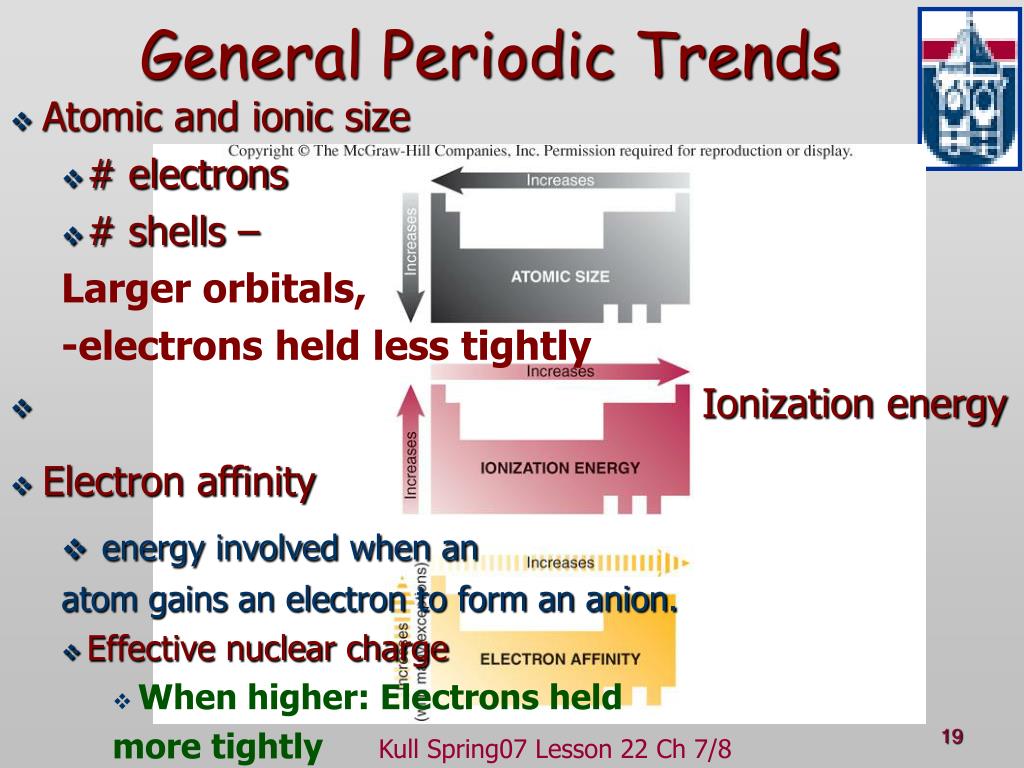
The effective nuclear charge increases from left to right in a period. Thus with the increase of the magnitude of effective nuclear charge, the magnitude of ionization potential also increases. The extent of penetration of valence electrons Effective Nuclear Charge: Greater the magnitude of effective nuclear charge, higher is the amount of energy needed to remove the outermost shell electron. Half filled and completely filled orbitals 6. Factors affecting the magnitude of Ionization Potential: 1. Low energies indicate ease of removal of electrons and vice versa. Ionization energies measure hoe tightly electrons are bound to atoms. Ionization energies are usually expressed in electron volts (eV) per atom or in kilojoules per mol (kJ/mol) 1eV/atom=96.48 kJ/mol Value of each ionization energy will increase with each removed electron, since the attractive influence of the nucleus increases and will and will require more energy for the removal of an electron from more positive charges. Second ionization energy (I2): amount of energy required to remove a second electron from the gaseous monopositive cation to form dipositive cation. First ionization energy (I1): amount of energy required to remove the most loosely bound electron from an isolated gaseous atom to form a cation. Ionization EnergyIonization Energy Ionization : The process of removing an electron from an isolated atom to form a positive ion. In this Assignment I will discuss the trends of Ionization potential and electron affinity in the periodic table. In general, properties correlate down a group of elements. The valence electron structure of atoms can be used to explain various properties of atoms. Periodic TrendsPeriodic Trends Ionization Energy, Electron Affinity and Atomic Radii. Ionization Potential And Electron Affinity Subject: Chemistry Course code: Chem-101 Submitted By: Aqsa Manzoor Discipline: Zoology Semester: I Roll No: 2180 Submitted To: Mrs. This is due to the gradual increase in the atomic size.Įlectro-negativity is the tendency of an atom to attract a shared pair of electrons more towards itself. The electro-negativity decreases gradually on moving down the group due to the corresponding increase in the atomic radii. These elements show higher values of ionisation enthalpy as compared to group 14 elements. This is due to their higher nuclear charge, smaller atomic radii and stable half-filled electronic configurations. As we move down the group, the value of ionisation enthalpy decreases.

Hence, the valence shell electronic configuration of these elements is ns2 np3.These elements have five electrons in their valence shell. Due to the exactly half-filled electronic configuration of the ‘n p’ subshell, the elements of this group are fairly stable.ĮX: Dinitrogen is an inert gas under normal conditions.Īs we move down the group, the atomic radii and ionic radii increase due to the addition of a new principal energy level in each successive element. The differentiating electron in these elements enters the n p subshell. The electronic configuration of group the fifteen elements: Phosphoproteins are present in egg yolk, bone marrow and milk. The remaining elements of the group, that is, arsenic, antimony and bismuth, mainly occur as sulphides.ĮX: Arsenopyrite, stibnite and bismuth glance. About 60 per cent of bones and teeth are composed of phosphates. Phosphate groups are constituents of nucleic acids, that is, DNA and RNA. Phosphorus is an essential constituent of plant and animal matter. It is the 11th most abundant element in the earth’s crust. In combined state, it occurs in the form of minerals as phosphates. EX: Chlorapatite, fluoroapatite and hydroxyapatite are some. It is also found as the essential constituent in proteins, amino acids, nucleic acids and enzymes. The next element in the group is phosphorus. Minerals of nitrogen: Chile saltpetre & Indian saltpetre.
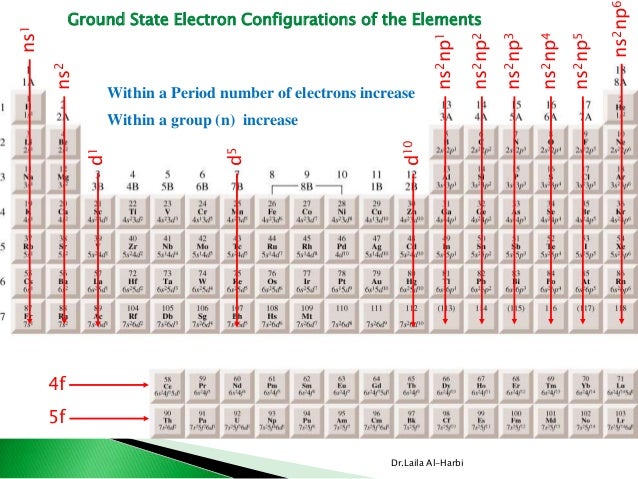
#Ns2 np3 valence shell free#
The elements in group fifteen are nitrogen, phosphorus, arsenic, antimony and bismuth. Nitrogen is the major constituent of the earth’s atmosphere, and accounts for 78 per cent of it by volume. It is the first member of this group, and occurs in free sate as a diatomic gas, N2. Although helium does not have p orbitals, it is a p-block element because it resembles that of other p-block elements of the eighteenth group with respect to their properties. Most p-block elements are non-metals, while the remaining are metals and metalloids. The general valence shell electronic configuration of these elements is ns 2, np 1-6. The electronic configuration of helium is 1s2. The p-block elements are placed to the right side of the periodic table in groups 13-18. In the atoms of p-block elements, the differentiating electron enters the valence p subshell. Thus, in these elements, the n p subshell is gradually filled.


 0 kommentar(er)
0 kommentar(er)
